Submitted by South Sound GREEN
In the face of COVID-19 and recent stay at home order, parents and guardians may find themselves looking for activities that not only keep students engaged, but also provide information about local environmental science and concerns. In our South Sound GREEN Home Based Science Project series, we will introduce and demonstrate various hands-on and at-home activities for children of all ages to do either indoors or outside!
 This time we continue learning about water quality by talking about the importance of pH!
This time we continue learning about water quality by talking about the importance of pH!
Acidification in Action
Grade Level: 5th-12th
Materials
- Two glass jars
- Water
- White vinegar
- Shells
- Mussel, oyster, and clam shells left over from your dinner plate will all work. Make sure the shells are empty and cleaned!
- If you don’t have shells available, you can use chalk.
Background
We’ve talked a lot about water quality in earlier articles, learning about dissolved oxygen in Soda Science, turbidity in Turbidity Trials, and nitrates in Nitrate Bingo. Today, we’re going to focus on a fourth water quality parameter, pH! pH, or potential of hydrogen, is technically a measure of hydrogen ions in a solution, but is more simply known as a relative measure of how acidic or basic a solution is. The pH scale goes from 0 to 14, with lower values being more acidic and higher values being more basic. Therefore, the middle of the scale, or a pH of 7, is considered neutral. Acids and bases have different properties that affect whatever they come in contact with, and knowing the pH of a solution is important to be able to handle it safely.
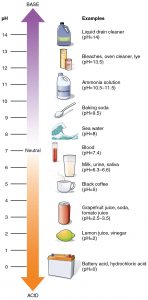
Common acids are generally sour, like lemon juice and vinegar. While these are usually safe for humans to interact with, stronger acids like battery acid can be very harmful if touched. Seawater is an example of a common base, though it is fairly weak. Bleach and lye, which are much stronger bases, are very dangerous for human interaction, just like strong acids. Humans tend to be safer around materials that are more neutral. Even our blood is close to a pH of 7!
Just like humans, aquatic organisms also tend to prefer neutral substances, and because they live in water with its own pH, any changes to the pH of their environment could have a large impact on the animals and plants that live there. Salmon generally prefer environments where the water has a pH between 7 and 8. If the pH is too low or too high, it can affect the layer of slime that protects salmon skin or even irritate the lenses of their eyes.. For animals that use shells, pH is even more important. Shells are often made from calcium carbonate, which is also what chalk is made of (chalk is actually the remains of millions and millions of small plankton with calcium carbonate shells!). Calcium carbonate is sensitive to pH changes and can break down in acidic environments. When pH decreases, shelled animals are not able to build their shells effectively and will struggle to survive. Recently, the waters of Puget Sound have gotten so acidic that oyster farms have shut down or have needed to relocate to safer waters (in some cases, going as far away as Hawaii!).
The process of marine waters becoming more acidic is known as ocean acidification. There are a lot of factors that influence ocean acidification, but the largest factor is increased atmospheric carbon dioxide. CO2 will increase the acidity of seawater when added (this is why soda water is a little more sour than still water), and with atmospheric CO2 levels being the highest now in human history, we’re seeing historically low pH levels in our oceans.
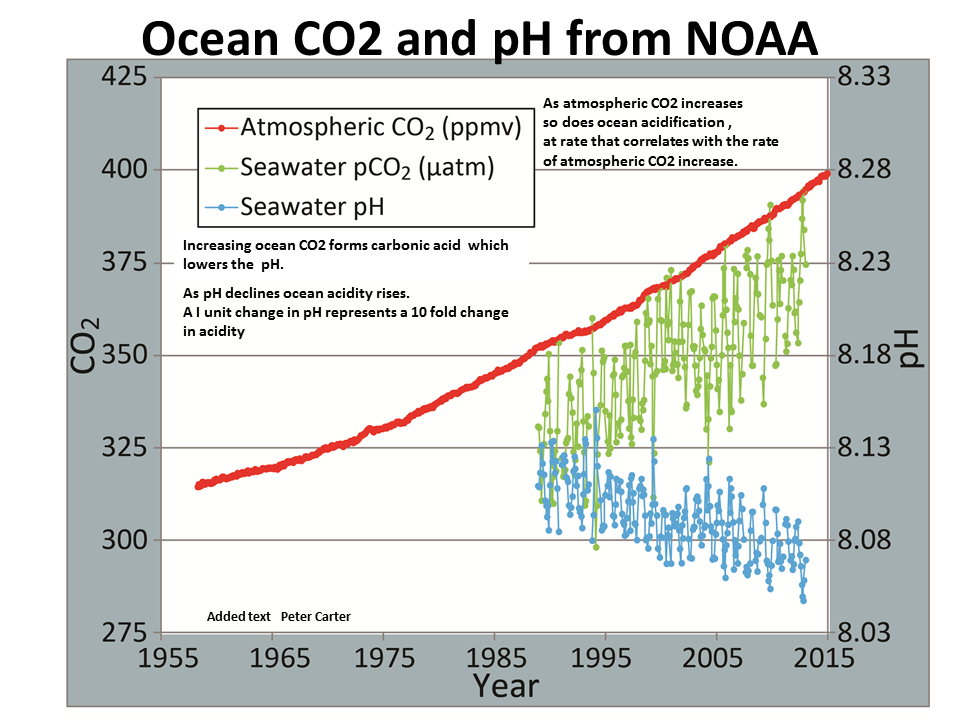
The impact of ocean acidification will not just harm marine life, but humans as well. Not to mention that many shelled animals help filter and clean our water! Without the regulation and reduction of global greenhouse gas emissions, ocean acidification will continue to have negative impacts on everything from oyster farming to whale watching, while also damaging our fragile oceans.
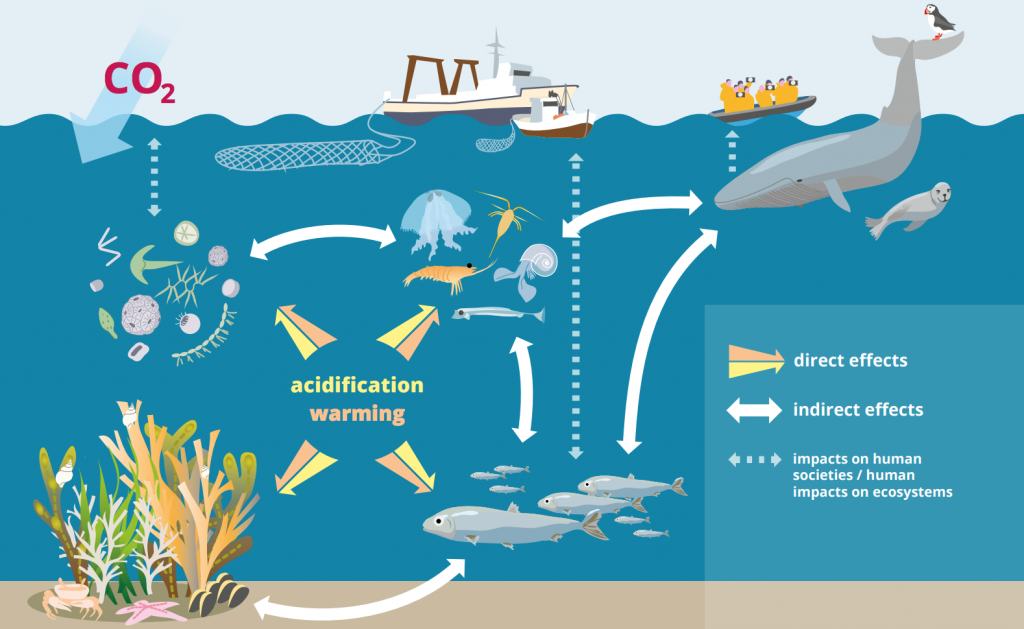
In this activity, you will see first-hand the effect of ocean acidification on shells by speeding up the process with a strong, household acid.
Procedure
This is a long-term experiment! Set up: 10 minutes. Duration: 1-2 weeks.
- Find a few shells – the best and safest way to do this is by getting them off of your dinner plate! Clam, oyster, and mussel shells will all work. If you cannot get shells, chalk will work as well.
- Clean the shells as thoroughly as possible before using in the experiment.
- Grab two clean glass jars, and put a shell (or multiple shells, if they’re small) or chalk into each jar.
- Fill one jar with water and the other jar with vinegar. Each jar should be filled enough so that the shells or chalk are all fully covered. Label each jar, but don’t worry if you forget – you’ll be able to smell the difference!

- Right away, you should notice bubbles forming on the shell(s) in the vinegar. Why might this be happening?

- Close each jar and let them rest for at least one week.
- After one week, check on your jars. Do you notice any differences between the shells?


- You can try the experiment again with different liquids in your home, or water from a nearby river or beach. Based on your results, do you believe the liquid is acidic, basic, or neutral?
Vocabulary
- Acidic: Describing a solution with a pH value below 7.
- Basic: Describing a solution with a pH value above 7. Also known as alkaline.
- Calcium Carbonate: A chemical that is used in the production of animal shells. Also known as chalk.
- Ocean Acidification: The process by which added atmospheric carbon dioxide entering the oceans leads to an overall decrease in pH level. This can have many harmful impacts on marine plants and animals.
- pH: The measurement of hydrogen ions in a solution.
Keep Learning!
- Learn more about ocean acidification with NOAA’s Ocean Acidification program
- Reduce ocean acidification with the NRDC!
- Share pictures of your projects with us on Instagram! Use the hashtag #GREENfromhome or find us at @southsoundgreen.
South Sound GREEN (Global Rivers Environmental Education Network) is a watershed education program in Thurston County that educates, empowers and connects thousands of local students in watershed studies annually. Through South Sound GREEN, participants engage in science and engineering practices related to water quality in South Sound. For more information, visit southsoundgreen.org.



















































